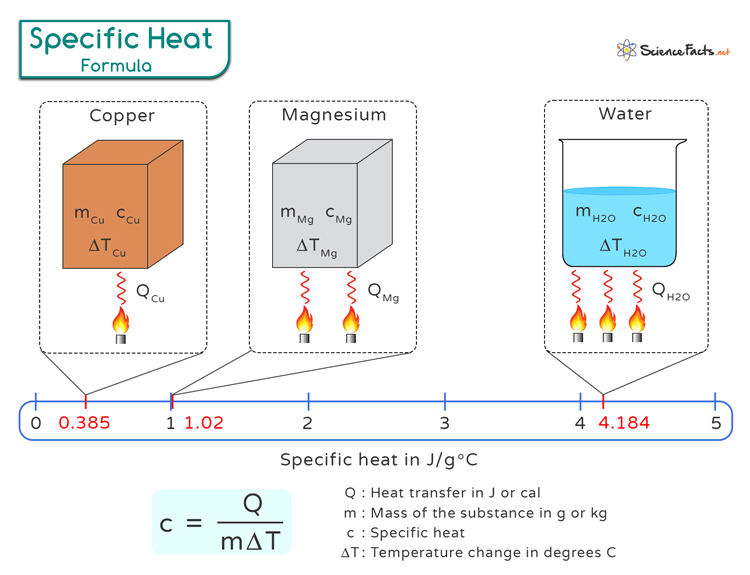
What Is Heat Capacity Relation . heat capacity is determined by both the type and amount of substance that absorbs or releases heat. It is usually expressed as calories. heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. Q = mcδt, where q is the. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. the quantitative relationship between heat transfer and temperature change contains all three factors: heat capacity, ratio of heat absorbed by a material to the temperature change. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree.
from www.sciencefacts.net
heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories. heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Q = mcδt, where q is the. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. the quantitative relationship between heat transfer and temperature change contains all three factors: heat capacity is determined by both the type and amount of substance that absorbs or releases heat. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it.
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems
What Is Heat Capacity Relation heat capacity, ratio of heat absorbed by a material to the temperature change. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. heat capacity, ratio of heat absorbed by a material to the temperature change. heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. Q = mcδt, where q is the. the quantitative relationship between heat transfer and temperature change contains all three factors: heat capacity is determined by both the type and amount of substance that absorbs or releases heat. It is usually expressed as calories. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Relation q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. It is usually expressed as calories. heat capacity, ratio of heat absorbed by a material to the temperature change. Q = mcδt, where q is the. heat capacity, also. What Is Heat Capacity Relation.
From www.slideserve.com
PPT Second Year Chemistry PowerPoint Presentation, free download ID806827 What Is Heat Capacity Relation the quantitative relationship between heat transfer and temperature change contains all three factors: heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a body is the quantity of heat required to. What Is Heat Capacity Relation.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Heat Capacity Relation It is usually expressed as calories. the quantitative relationship between heat transfer and temperature change contains all three factors: heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. heat capacity is determined by both the type and amount of substance that absorbs or releases heat. heat capacity,. What Is Heat Capacity Relation.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free download ID5567619 What Is Heat Capacity Relation heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories. Q = mcδt, where q is the. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. heat capacity, also known as thermal capacity, is a physical property of matter. What Is Heat Capacity Relation.
From www.slideserve.com
PPT Heat capacity PowerPoint Presentation, free download ID1419022 What Is Heat Capacity Relation heat capacity, ratio of heat absorbed by a material to the temperature change. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Q = mcδt, where q is the. the heat capacity of a substance describes how its. What Is Heat Capacity Relation.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Relation It is usually expressed as calories. heat capacity is determined by both the type and amount of substance that absorbs or releases heat. Q = mcδt, where q is the. heat capacity, ratio of heat absorbed by a material to the temperature change. the quantitative relationship between heat transfer and temperature change contains all three factors: . What Is Heat Capacity Relation.
From studylib.net
Heat Equation What Is Heat Capacity Relation It is usually expressed as calories. heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. the heat capacity of a substance describes how its temperature changes as it absorbs or. What Is Heat Capacity Relation.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is Heat Capacity Relation heat capacity is determined by both the type and amount of substance that absorbs or releases heat. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the quantitative relationship between heat transfer and temperature change contains all three. What Is Heat Capacity Relation.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Relation It is usually expressed as calories. heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. Q = mcδt, where q is the. heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a body is the quantity of heat required to raise. What Is Heat Capacity Relation.
From www.youtube.com
Heat Capacity at Constant Volume (Cv) and at Pressure(Cp) Relation YouTube What Is Heat Capacity Relation the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. It is usually expressed as calories. heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m. What Is Heat Capacity Relation.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is Heat Capacity Relation The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance,. What Is Heat Capacity Relation.
From www.slideserve.com
PPT CHAPTER 19 THERMAL PROPERTIES PowerPoint Presentation, free download ID6722582 What Is Heat Capacity Relation heat capacity is determined by both the type and amount of substance that absorbs or releases heat. It is usually expressed as calories. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. heat capacity, ratio of heat absorbed by a material to the temperature change. the quantitative. What Is Heat Capacity Relation.
From www.studypool.com
SOLUTION Heat capacities of an ideal gas at constant pressure and volume and relation between What Is Heat Capacity Relation The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Q = mcδt, where q is the. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. It is usually expressed as. What Is Heat Capacity Relation.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is Heat Capacity Relation It is usually expressed as calories. the quantitative relationship between heat transfer and temperature change contains all three factors: heat capacity, ratio of heat absorbed by a material to the temperature change. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. heat capacity is determined by both. What Is Heat Capacity Relation.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Is Heat Capacity Relation heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. Q = mcδt, where q is the. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. heat capacity, ratio of. What Is Heat Capacity Relation.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Is Heat Capacity Relation heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. heat capacity is determined by both the type and amount of substance that absorbs or releases heat. Q = mcδt, where q is the. q. What Is Heat Capacity Relation.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free download ID5567619 What Is Heat Capacity Relation the quantitative relationship between heat transfer and temperature change contains all three factors: heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. Q = mcδt, where q is the. . What Is Heat Capacity Relation.
From physics.stackexchange.com
thermodynamics Derivation of heat capacity at constant pressure and temperature Physics What Is Heat Capacity Relation heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of. heat capacity is determined by both the type and amount of substance that absorbs or releases heat. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and. What Is Heat Capacity Relation.